ATTENTION ALL CUSTOMERS:
Due to a recent change in our pharmacy software system, all previous login credentials will no longer work.
Please click on “Sign Up Today!” to create a new account, and be sure to download our NEW Mobile app!
Thank you for your patience during this transition.
Manténgase sano!
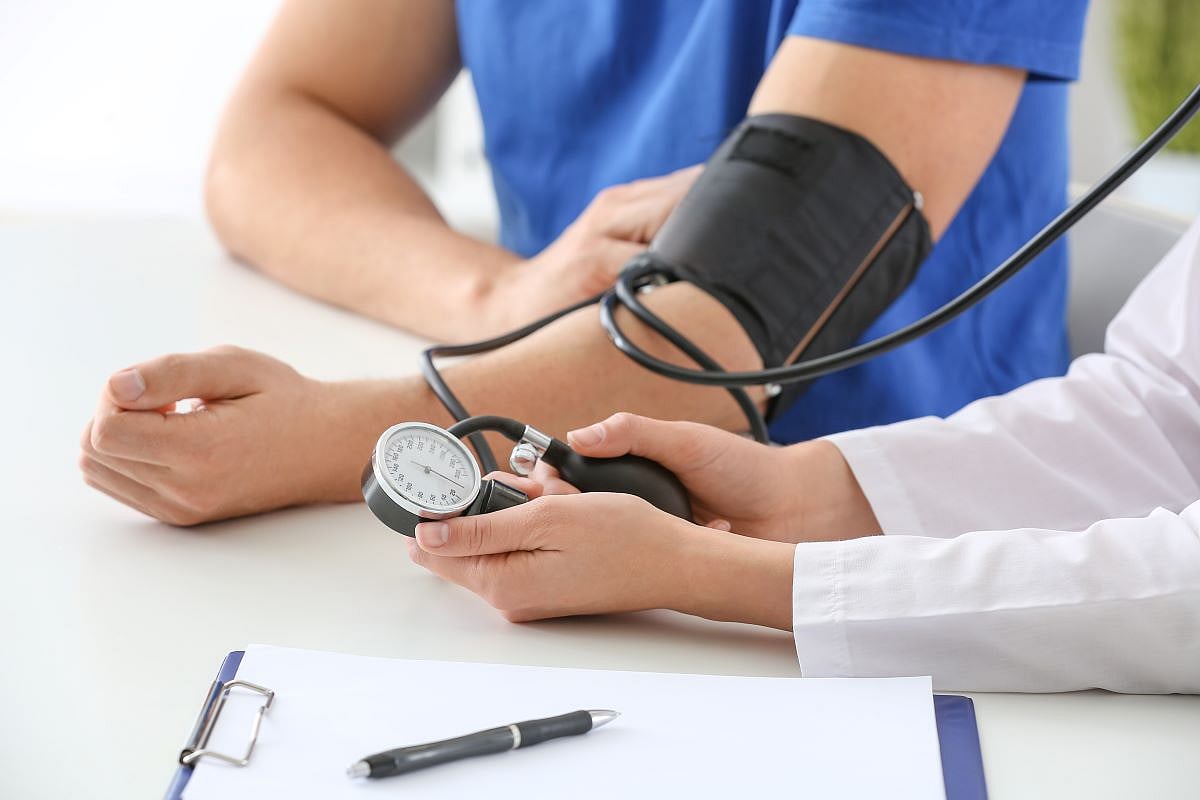
- Posted September 4, 2024
3-in-1 Blood Pressure Pill Could Be Treatment Advance
An experimental three-in-one blood pressure pill works better than layering on meds one at a time, a new clinical trial shows.
After a month on the combo pill, 81% of patients had their blood pressure under control compared with 55% of patients receiving standard care, researchers report.
“The triple pill still produced clinically meaningful reductions in blood pressure compared to standard care, even when standard care closely followed current guidelines and involved more clinic visits,” said lead investigator Dr. Dike Ojji, head of the Cardiovascular Research Unit at the University of Abuja in Nigeria.
“In low-income countries, fewer than one in four treated people achieve blood pressure control, and in high-income settings it is only between 50% and 70%, so to see rates of over 80% in just one month is impressive,” Ojji added.
The GMRx2 pill, which was developed by the pharmaceutical company George Medicines, contains the blood pressure meds telmisartan, amlodipine and indapamide. It’s taken once daily. The company is part of the George Institute for Global Health.
Researchers compared people taking the combo pill to those receiving standard treatment for high blood pressure, which involves starting off with one drug and then adding on others.
Systolic blood pressure was 31 points lower in the combo pill group after six months of treatment, compared to 26 points lower with standard care, results showed.
The five-point systolic difference is equal to a 10% reduction in stroke, heart attack and heart failure risk between the two groups, researchers said in a George Institute news release.
It’s estimated more than a billion people worldwide have high blood pressure, which accounts for nearly 11 million deaths each year, researchers said in background notes.
George Medicines has submitted the combo drug to the U.S. Food and Drug Administration for approval.
The trial results were presented Saturday at the European Society of Cardiology’s annual meeting in London and published simultaneously in the Journal of the American Medical Association. Positive data from two other trials of the combo pill were also presented at the meeting.
More information
The American Heart Association has more on managing high blood pressure.
SOURCE: George Institute for Global Health, news release, Aug. 31, 2024






