ATTENTION ALL CUSTOMERS:
Due to a recent change in our pharmacy software system, all previous login credentials will no longer work.
Please click on “Sign Up Today!” to create a new account, and be sure to download our NEW Mobile app!
Thank you for your patience during this transition.
Get Healthy!
- Posted September 20, 2023
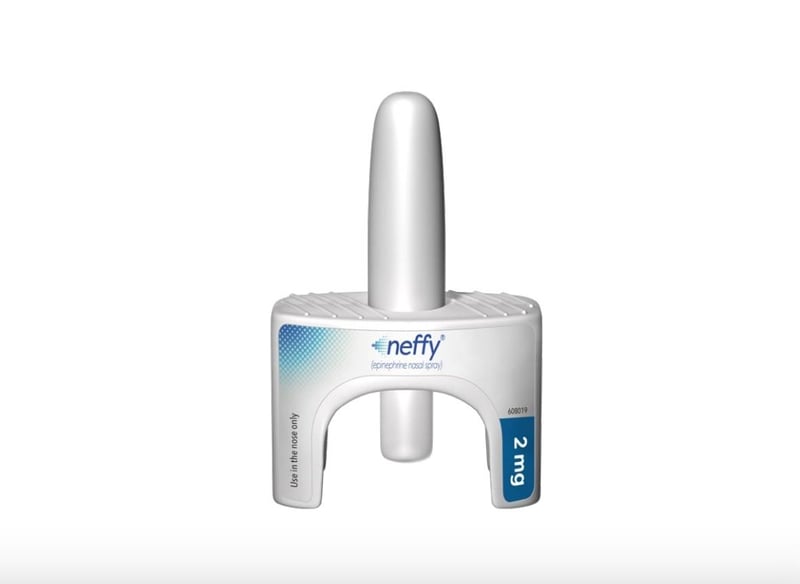
FDA Wants More Data on First Needle-Free Antidote for Severe Allergic Reactions
In a surprising move, the U.S. Food and Drug Administration (FDA) has opted not to approve a needle-free alternative to the EpiPen for emergency treatment of severe allergic reactions.
Approval of the Neffy nasal spray was widely anticipated. An FDA advisory panel voted to recommend approval of the drug for children and adults in May. While the FDA is not obligated to follow the advice of their advisory panels, it usually does.
Instead, the FDA told the drug's maker, ARS Pharmaceuticals, that it needed to conduct another study on the drug before it is approved, the company said in a statement late Tuesday night.
"We are deeply disappointed that this action further delays the availability of Neffy for the millions of people who are at risk of a potentially life-threatening severe allergic reaction," said Richard Lowenthal, co-founder, president and CEO of ARS Pharma.
"We stand by the totality of the Neffy data package in a comprehensive registration program that was aligned upon with FDA and believe strongly in the value Neffy can provide for patients, families and caregivers living daily with severe allergic reactions," he said in a company statement, adding that his firm will aim to complete the requested trial as soon as possible.
The news was unwelcome on the front lines of health care.
"It's certainly disappointing as we were hoping to have another option for people at risk of severe allergic reactions," said Dr. Scott Sicherer, director of the Elliot and Roslyn Jaffe Food Allergy Institute at Mount Sinai in New York City.
Both Neffy and EpiPen deliver epinephrine, which works by relaxing the muscles in the airways. Neffy is a nasal spray, while EpiPen is given as an injection into a big muscle, such as the thigh.
During an anaphylactic reaction, symptoms such as trouble breathing and swallowing, hives, nausea and vomiting can come on suddenly when a person is exposed to a triggering food, medication or insect sting. Administered quickly, epinephrine can stop this potentially fatal cascade of symptoms.
"Neffy would have been the first needle-free option for patients to treat severe allergic reactions, but we still have the injectable epinephrine devices available,"said Dr. Payel Gupta, an assistant clinical professor at SUNY Downstate Medical Center in New York City and volunteer medical spokeswoman for the American Lung Association. "A needle-free option would be great for people with needle phobia or who are anxious about injecting themselves."
The bottom line is that having epinephrine available is vital.
"Epinephrine is a lifesaving medication and every patient who has a [history of potentially severe] allergy needs to carry the device at all times," Gupta said. "Experts recommend carrying two EpiPens as sometimes one dose isn't enough."
Sicherer agreed.
If you think you are experiencing an allergic reaction, use your epinephrine device, he said.
"Do not wait for trouble breathing or passing out,"Sicherer said. "The medication is safe, effective and while it may cause a tiny ouch, it could be lifesaving."
More information
Learn what to do during a severe allergy attack.
SOURCES: Scott Sicherer, MD, director, Elliot and Roslyn Jaffe Food Allergy Institute, division chief, allergy and immunology, Department of Pediatrics; Mount Sinai Health System, New York City; Payel Gupta, MD, assistant clinical professor, SUNY Downstate Medical Center, New York City, volunteer medical spokeswoman, American Lung Association; ARS Pharmaceuticals, news release, Sept. 19, 2023






